Production Technology of Highly Unsaturated Fatty Acids as Fish Oil Substitute Utilizing Microalgae
Introduction
Japan's fisheries industry relies heavily on natural resources, with about 75% of production coming from fishing and only around 25% from aquaculture (Fisheries Agency, 2020). Globally, the aquaculture sector has been growing significantly (Fisheries Agency, 2020). However, aquaculture also depends on substantial amounts of fishmeal and fish oil due to the nutritional requirements of fish. Thus, even in aquaculture, the foundation remains reliant on marine resources. The main sources of fishmeal and fish oil are from abundant small pelagic fish, particularly the Peruvian anchoveta resource. As aquaculture production grows, so does the demand for fishmeal and fish oil. The international price of fishmeal tripled between 2002 and 2010 (NOAA/USDA, 2011). Given this context and considering the perspective of the Sustainable Development Goals (SDGs) - specifically goals 12 and 14 - and the need to address the rising costs of fishmeal and fish oil, there is a push for the development of alternative feed materials. Various materials are being researched for this purpose. In this study, we introduce research on microalgae as a substitute for fish oil and highlight our own efforts in this field.
Fishmeal, Fish Oil, and New Material Options
Alternatives to fishmeal include plant-based materials such as soybean meal and corn gluten meal, as well as animal-based materials like feather meal, chicken meal, and blood meal. Insects like mealworms and black soldier flies, as well as microbial sources like hydrogen bacteria and yeast, are also under consideration. However, these materials are primarily focused on substituting fishmeal protein, not fish oil. Fish oil contains essential n-3 polyunsaturated fatty acids (n-3PUFA) such as EPA and DHA. These are crucial nutrients for the growth of fish, particularly marine species (Sato, 2009). EPA and DHA are also essential for human health and are significant motivators for global seafood consumption (FAO/WHO, 2010). Anticipated future demand for n-3PUFA for aquaculture and human consumption is expected to surpass the supply from marine sources (NOAA/USDA, 2011). Microalgae are reported to be the only viable candidates for fish oil substitution among the various material options (Cottrell et al., 2020).
What is Aurantiochytrium?

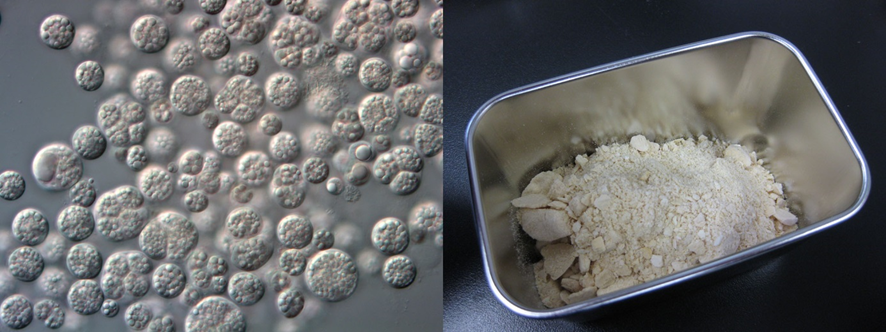
Figure 1: Microscopic Image of Aurantiochytrium limacinum NIES-3737 Strain (Left)
and Dried Algal Biomass (Right)
The microalgae introduced in this article belong to the Aurantiochytrium genus. Aurantiochytrium (Aurantiochytrium sp.) (Figure 1) is a genus of microalgae belonging to the Labyrinthulales class. It was separated from the Schizochytrium genus in 2007 (Yokoyama and Honda, 2007). Aurantiochytrium is a heterotrophic organism that doesn't perform photosynthesis and grows on organic substrates like bacteria and yeast. In nature, it's often found in nutrient-rich environments like mangrove swamps in marine waters (Yoshida, 2016). One of the distinctive features of Aurantiochytrium is its ability to synthesize and accumulate large amounts of n-3PUFA, particularly DHA. Additionally, some strains of Aurantiochytrium accumulate significant lipid content, with up to 50% or more of the dry weight being lipids. Because of its heterotrophic nature, it can be cultured at high densities in tanks without relying on sunlight, making it advantageous for mass cultivation.
Application Research of Aurantiochytrium in Fish Feeds under SIP
From 2014 to 2018, we conducted research on the application of Aurantiochytrium in fish feeds as part of the Strategic Innovation Program (SIP). Collaborating with the University of Tsukuba, Euglena Co. Ltd., Higashimaru Co. Ltd., and others, we investigated the use of Aurantiochytrium in fish feeds. Euglena Co. Ltd. developed mass cultivation techniques, the University of Tsukuba focused on fundamental research of cultivation techniques, Higashimaru Co. Ltd. conducted fish rearing trials, and the Fisheries Research Agency analyzed the algae and conducted initial feed studies. Euglena Co. Ltd. cultivated Aurantiochytrium limacinum, NIES-3737 strain (Figure 1), selected for its growth rate and lipid content using their mass cultivation technology. The dried algal biomass was analyzed by the Fisheries Research Agency, revealing lipid content consistently exceeding 50%, with over 30% of those lipids being DHA. EPA content was low, comprising around 0.2% of total fatty acids (Figure 2). Protein content was around 10%. In some cases, lipid content reached nearly 70%, with dried algal powder containing over 20% DHA, presenting a valuable alternative not only as feed but also for functional food applications.
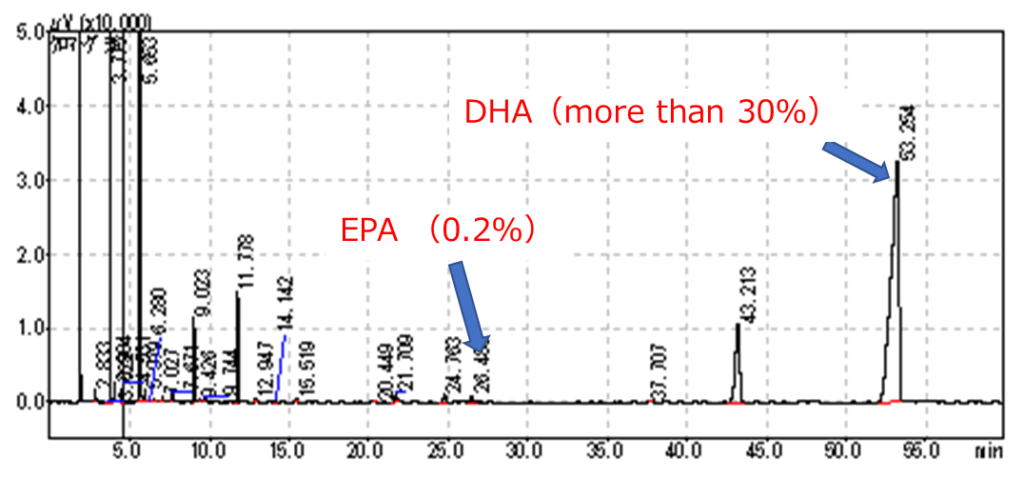
Figure 2: Example of Fatty Acid Analysis for NIES-3737 Strain The lipid content in dried algal biomass was approximately 51%, with DHA constituting 33% of the lipids and EPA at 0.2%.
In the SIP program, our research mainly focused on food applications of Aurantiochytrium, and since DHA was the primary n-3PUFA found in its composition with low EPA content, the emphasis was on enhancing DHA levels in farmed fish rather than replacing fish oil. Higashimaru Co. Ltd. conducted trials by adding Aurantiochytrium to conventional feed formulations, achieving DHA enhancement of 20% to 50% in species such as kuruma shrimp, yellowtail, greater amberjack, abalone, and filefish (Figure 3).

Figure 3: DHA Enrichment in Farmed Fish with Aurantiochytrium-Added Feed Enrichment of DHA in farmed fish was achieved through the administration of Aurantiochytrium-added feed. No adverse effects on growth or other factors were observed.
Aurantiochytrium Research in Moonshot Project
Although SIP Phase 1 concluded in 2018, practical application research on Aurantiochytrium continued diligently by Euglena Co. Ltd. Furthermore, research on using Aurantiochytrium as a fish oil substitute has been conducted in species like yellowtail (Fukada et al., 2020) and greater amberjack (Seong et al., 2020). Starting in 2020, we initiated research on Aurantiochytrium within the Moonshot Project for "Developing an Insect-Based Circular Food Production System to Solve Global Food Issues and Support Human Space Exploration." Collaborating institutions include the National Agriculture and Food Research Organization (NARO), Fisheries Research Agency, Euglena Co. Ltd., Shizuoka Prefectural Research Institute of Fisheries and Aquaculture, University of Tsukuba, among others. While this project aims to apply insect resources like crickets and mealworms for food and feed, the lack of EPA and DHA in these insect sources necessitates the use of microalgae like Aurantiochytrium to optimize feed formulations. By replacing fishmeal with insect resources and fish oil with microalgae, such as Aurantiochytrium, we can potentially create a "fully circular feed" that is independent of natural resources. However, our aspirations go further. Approximately half of the domestically produced fishmeal and fish oil in Japan are derived from urban and fisheries processing residues, making them quite "sustainable" (Japan Marine Products Association, 2015). Moreover, as mentioned earlier, various alternatives to fishmeal exist. Therefore, we aim to collect data on the suitability of each candidate feed material and use data-driven methodologies to create a model that determines the "optimal blend" for feed formulation, contributing to the development of truly sustainable and cost-effective fish feeds.
Acknowledgments :
Some of the data presented in this article are derived from the results of the Strategic Innovation Program Phase 1, "Next-Generation Agricultural, Forestry and Fisheries Industry Creation Technology." While not all contributors could be included as co-authors, we are deeply grateful to everyone involved in this research. Additionally, the Moonshot Project, "Developing an Insect-Based Circular Food Production System to Solve Global Food Issues and Support Human Space Exploration," is supported by the Japan Agency for Medical Research and Development (AMED) through the Systematic Creation of a Sustainable and Regenerative Society project (JPJ009237). We extend our gratitude to all those involved.
References:
Cottrell, R.S. et al. (2020) Global Adaptation of Novel Aquaculture Feeds Could Substantially Reduce Forage Fish Demand by 2030. Nature Food 1: 301-308.
FAO/WHO (2010) Report of Joint FAO/WHO Expert Consultation on the Risks and Benefits of Fish Consumption.
Fukada, H. et al. (2020) Effects of complete replacement of fish oil with plant oil mixtures and algal meal on growth performance and fatty acid composition in juvenile yellowtail Seriola quinqueradiata. Fish. Sci. 86: 107-118.
NOAA/USDA (2011) The Future of Aquafeeds.
Seong,T. et al. (2020) Non-fish meal, non-fish oil diet development for red sea bream, Pagrus major, with plant protein and graded levels of Schizochytrium sp.: Effect on growth and fatty acid composition. Aquaculture Nutrition 26: 1173-1185.
Yokoyama, R and Honda, D (2007) Taxonomic rearrangement of the genus Schizochytrium sensu lato based on morphology, chemotaxonomic characteristics, and 18S rRNA gene phylogeny (Thraustochytriaceae, Labyrinthulomycetes): emendation for Schizochytrium and erection of Aurantiochytrium and Oblongichytrium gen. nov. Mycoscience 48: 199-211.
Sato, S. (2009) Fish Nutrition and Feeds (in Japanese). pp. 120-131.
Fisheries Agency (2020) Fisheries White Paper for FY2020 (in Japanese).
Japan Marine Products Association (2015) JMOA Report No. 11 (in Japanese).
Yoshida, M. (2016) Hydrocarbon Production by Aurantiochytrium. Kaiyo to Seibutsu (Marine and Organism) 38: 46-48.
